Liquid-liquid Interfacial Tensions
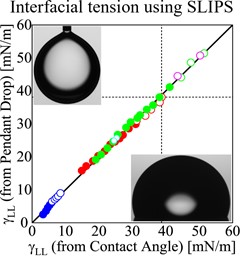
Similar to surface tension, the interfacial tension between two immiscible liquids is the work needed to increase the size of their mutual interface. There are a range of methods to measure liquid-liquid interfacial tensions with one of the most common being the pendant drop method [1]. When a droplet hangs from a capillary in another liquid of a different density, it elongates due to gravitational forces. By measuring the shape of the interface between the droplet and the surrounding liquid, it is possible to deduce the liquid-liquid interfacial tension.
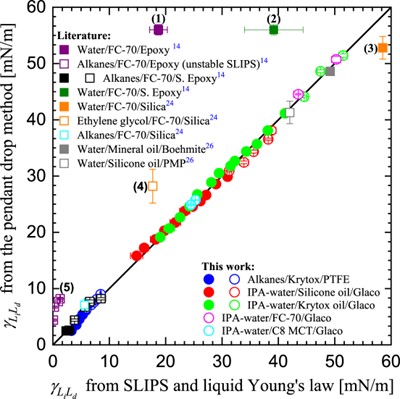
The liquid Young’s law provides a new method of making estimates of liquid-liquid interfacial tensions simply by measuring contact angles of droplets on surfaces in air [2]. The only interfacial tensions in the liquid Young’s law are the droplet-vapor and lubricant-vapor surface tensions, and the droplet-lubricant liquid-liquid interfacial tension. The droplet liquid-lubricant liquid interfacial tension can be deduced from the contact angle of a droplet on a continuous thin lubricant layer. This method gives excellent results comparable to the pendant drop method provided the droplet liquid does not displace the lubricant liquid on the underlying solid as shown in the graph below.
Publications
- Boundary tension by pendant drops. J.M. Andreas, E.A. Hauser and W.B. Tucker, Journal of Physical Chemistry 42 (1938), 1001–1019.
- The liquid Young’s Law on SLIPS: Liquid–liquid interfacial tensions and Zisman plots. G. McHale, N. Afify, S. Armstrong, G.G. Wells and R. Ledesma‐Aguilar, Langmuir 38 (2022), 10032–10042.
