The Liquid Surface Zisman Plot
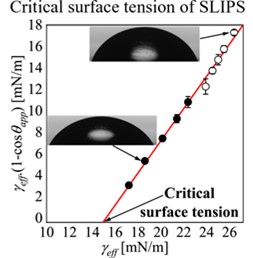
In 1964, William Zisman discovered empirically that plotting the cosine of the contact angle against the surface tension for a homologous series of alkane droplets on a solid surface gave a straight line [1]. He defined the Critical Surface Tension (CST) as the highest liquid surface tension that can completely wet a specific solid surface. Zisman extended his method to using non-homologous liquids to measure the critical surface tension. This discovery revolutionized adhesive bonding and surface chemistry because for adhesive bonding complete wetting is used to maximize the adhesive joint strength.
By extending the concept of wettability of a solid surface to wettability of a thin liquid surface, it is possible to create a Zisman plot for a SLIP surface [2]. In this case, the extrapolation of the data to give complete wetting (a contact angle of zero degrees) provides a Critical Surface Tension for the specific lubricant liquid.
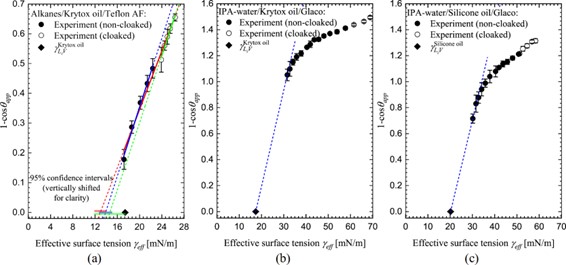
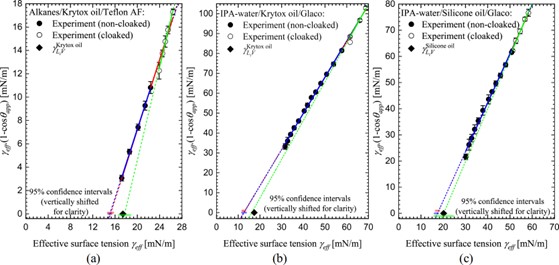
This method works well for alkanes provided the droplet liquid does not displace the lubricant liquid. For non-homologous liquids, such as mixtures of isopropyl alcohol and water, the surface tension range is much greater and extrapolating the data to a contact angle of zero becomes difficult. However, a modified Zisman plot [3] can be used as shown in the graph below. In these plots the surface tension of the lubricant is an upper bound on the Critical Surface Tension.
Publications
- Relation of the equilibrium contact angle to liquid and solid constitution. W.A. Zisman in Contact Angle, Wettability, and Adhesion; 1964; pp 1–51.
- The liquid Young’s Law on SLIPS: Liquid–liquid interfacial tensions and Zisman plots. G. McHale, N. Afify, S. Armstrong, G.G. Wells and R. Ledesma‐Aguilar, Langmuir 38 (2022), 10032–10042.
- Contact angle patterns on low-energy surfaces. R. David and A.W. Neumann, Advances in Colloid and Interface Science 206 (2014), 46–56.
