Superhydrophobic surfaces are those upon which water droplets will ball up with a high contact angle. They will roll off the surface when it is tilted at even a small angle. Inspired by the Lotus leaf and other plants and animals in the natural world, we can make artifical superhydrophobic surfaces in a variety of different ways. Some of these methods utilise surface chemistry to generate superhydrophobicity, whereas others use topography.
The following are some examples of artificial superhydrophobic surfaces.
Polymer
Microposts
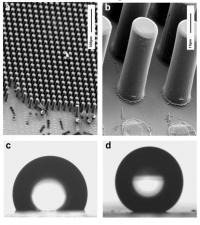
Photolithography can be used to create pillars like a ‘bed of nails’ but on a much smaller length scale. These surfaces are useful for comparing observed superhydrophobic effects to theoretical predictions.
Fractal
Structures

Surfaces can be grown in which deposition follows a process called diffusion limited aggregation. When treated with a hydrophobic coating, droplets of water completely ball up and roll off these surfaces.
Etched
Copper
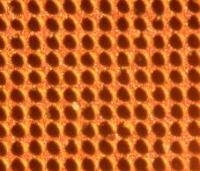
‘Craters’ can be etched into a Copper surface until they join up to create a landscape of sharp pinnacles.
Multi-scale
Texturing
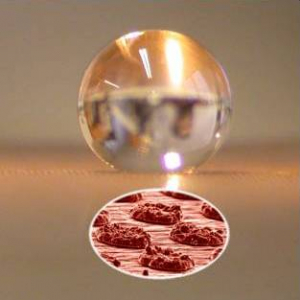
Just as the lotus leaf has small bumps on large bumps, photolithographic techniques can overlay large-scale textured surfaces with small-scale roughness.
Granular
Materials

Grains of sand can be given a hydrophobic coating to create a superhydrophobic surface which water rolls off.
There are two other examples of structures that use similar principles to superhydrophobicity:
Liquid Marbles

If we have a surface consisting of superhydrophobic grains that are loose rather than fixed (as was the case in the example of superhydrophobic sand), the grains can stick to the surface of a water droplet. This creates a ‘liquid marble’ that can roll freely on solid (and water) surfaces.
Leidenfrost Effect
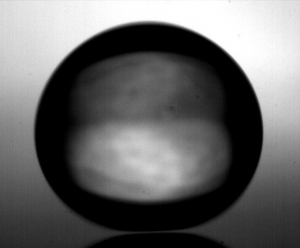
When water droplets are placed on a very hot surface, they can remain as droplets for some considerable time because a layer of vapour between the droplet and the surface insulates the droplet from the hot surface. This is known as the Leidenfrost effect, as we discussed here. Here, we show some videos illustrating involving the Leidenfrost effect.

