Research involving liquid-like surfaces
The flexibility of a polymer chain depends on whether the temperature is above or below the polymer’s glass transition temperature, Tg. Above Tg, the chain is in a liquid-like state unless cross-linking or interactions with other chains causes it to lose that flexibility. Polydimethylsiloxane (PDMS) has an extremely low Tg (below 100 ∘C) and if attached to a surface using short chains at the optimum density to create “mushroom” conformations leads to a hydrophobic surface with liquid-like properties on which droplets (and contact lines) can easily move.
Below you can read about some other work of this group related to liquid-like surfaces.
Evaporation on slippery liquid-like surfaces
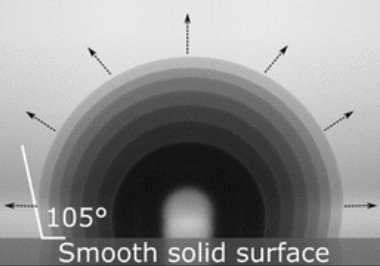
When a droplet evaporates from a slippery liquid-like surface, its contact area smoothly retracts as its volume reduces.
Droplet and contact-line friction

We can use a surface coating to change kinetic contact line friction, which dramatically alters how quickly droplets move on a surface.
Anti-biofilm liquid and liquid-like surfaces
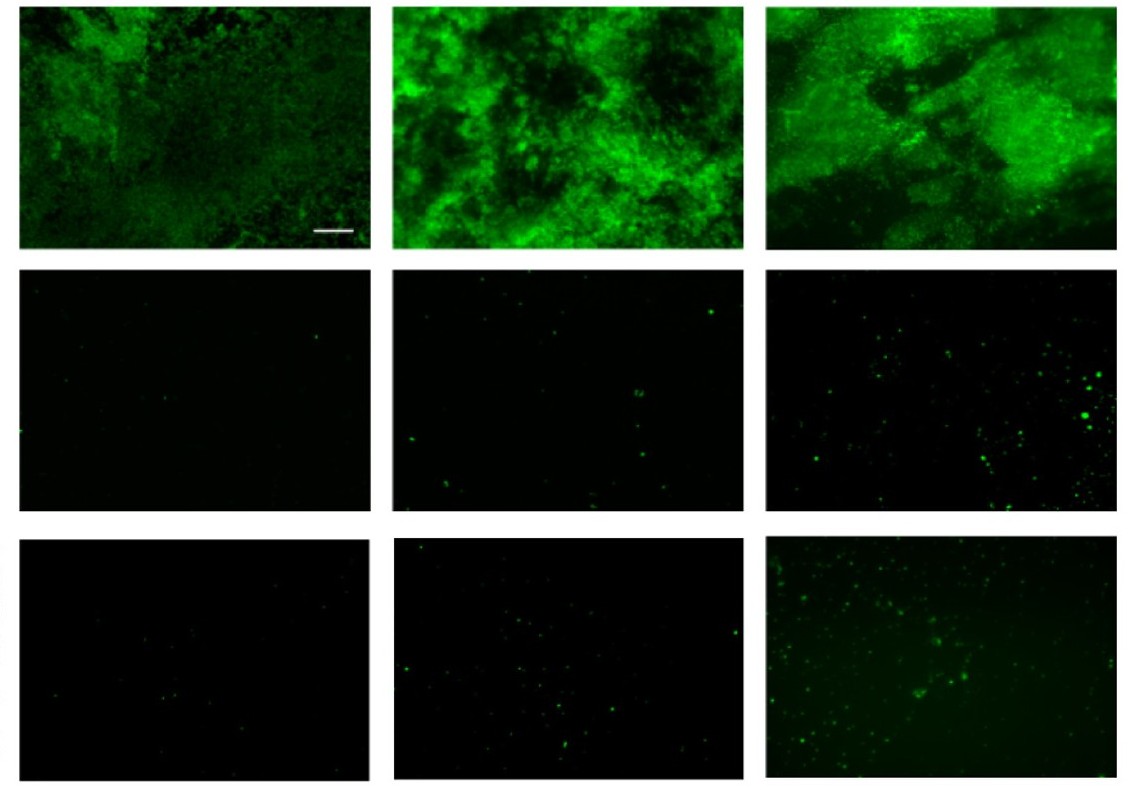
Unwanted biofilms often form on surfaces, such as bacterial growth on medical devices. Making the surfaces of the device slippery through a solid or liquid lubricant can reduce biofilm formation.
Anti-icing liquid-like surfaces

Ice formation in the natural environment is dangerous when it occurs on surfaces such as on planes, power lines and bridges. Creating liquid-like surfaces can produce resistance to this icing. (Image of ice on a turbine blade from NASA.)
Anti-scaling liquid-like surfaces
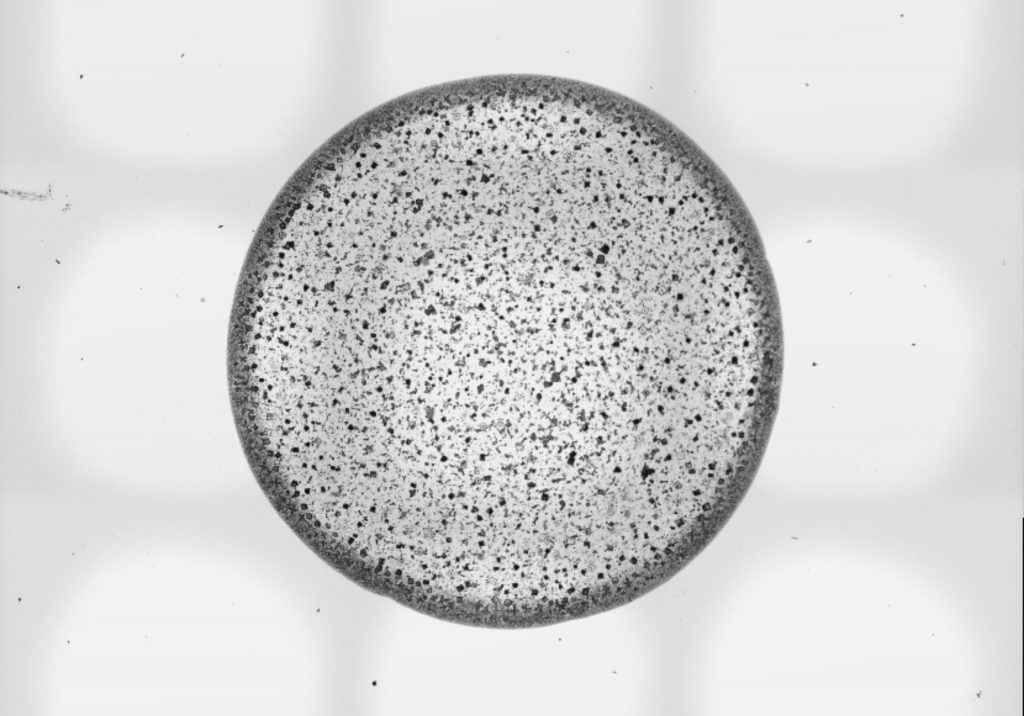
When water evaporates from a surface, it leaves contaminants which over time will accumulate and cause fouling, blockages and corrosion. Surfaces engineered to be slippery to liquids can suppress the deposition of salt.
