Evaporating Droplets
As a droplet evaporates, the curvature becomes greater, which leads to a greater pressure inside the droplet. When the droplet rests on a patterned surface, this leads to a sudden transition to a state in which the water penetrates between surface protrusions (‘nails’). This is known as a Cassie-to-Wenzel transition. The reduction in contact angle is illustrated schematically below, where we can see that ϴW is smaller than ϴCB.
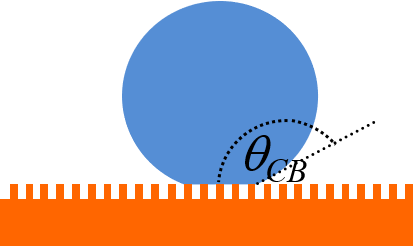
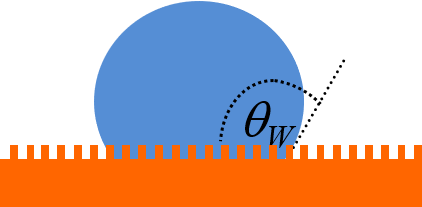
The video below shows a suspended-to-collapsed transition during droplet evaporation from a patterned substrate.
Publication
Analysis of droplet evaporation on a super-hydrophobic surface G. McHale, S. Aqil, N.J. Shirtcliffe, M.I. Newton and H.Y. Erbil, Langmuir 21 (2005)
