Anti-scaling Liquid-like Surfaces
Water is essential to life and in industry is used to cool surfaces. But when water evaporates, the contaminants and minerals within it will precipitate and accumulate on the solid surface of the substrate or container causing fouling, blockages and corrosion [1]. Engineering a surface to avoid salt forming on the substrate means controlling when crystallization from the solution occurs.
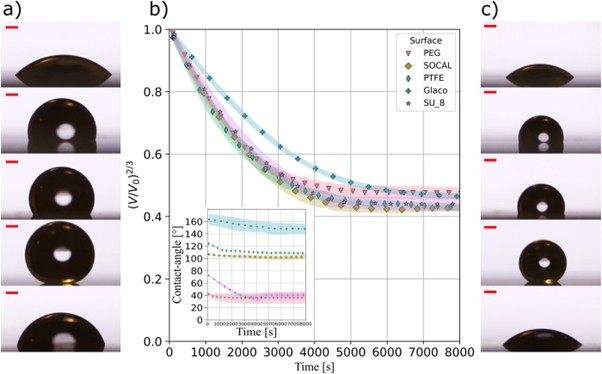
Droplet of water do not always completely evaporate if they contain sugar or salt – a well-known and used principle in food preservation. The figure above shows how salt solution droplets on different surfaces reduced in volume due to evaporation and so increase the salt concentration until a stable droplet volume is reached. In the equilibrium state the salt solution can become supersaturated and contact with a surface can cause crystallization and deposition of salt crystals. Surfaces engineered to be slippery to liquids, such as superhydrophobic (Glaco) and hydrophobic (SOCAL) and hydrophilic (PEG) liquid-like surfaces, remove contact line pinning and suppress the deposition of salt [2].
Publications
- Crystal critters: Self-ejection of crystals from heated, superhydrophobic surfaces S. A. McBride, H.-L. Girard and K. K. Varanasi, Science Advances 7 (2021), 1–9
- Suppression of crystallization in saline drop evaporation on pinning-free surfaces A. Jenkins, G. G. Wells, R. Ledesma-Aguilar, D. Orejon, S. Armstrong and G. McHale, The Journal of Chemical Physics 158 (2023), 124708
