Super-slippery surfaces
Some surfaces can be such that water forms a continuous film which acts as a lubricating layer. These water-attracting surfaces are called superhydrophilic (rather than superhydrophobic). The best example of this in nature is the Nepenthes pitcher plant, which is very slippery to the oily feet of ants and other insects when the surface of the pitcher plant is wet. The insects then fall into the pitcher and can’t escape.

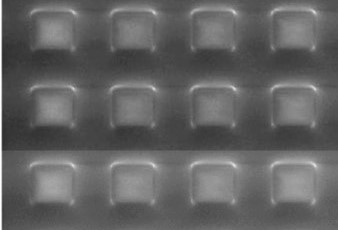
We can follow the same ideas as those employed by the Nepenthes pitcher plant to manufacture artificial surfaces that are super-slippery by coating a textured surface with a lubricant. This lubricant can fill the spaces in the patterned surface, as in the image on the left below. However, the most slippery surface is when the liquid forms a continuous coating across the whole surface, as in the image on the right. In both cases, the lubricant may spread partially up the sides of the drop, or it may fully cloak the drop. The result is a slippery liquid-infused porous surface (SLIPS) that is very slippery to other immiscible liquids.
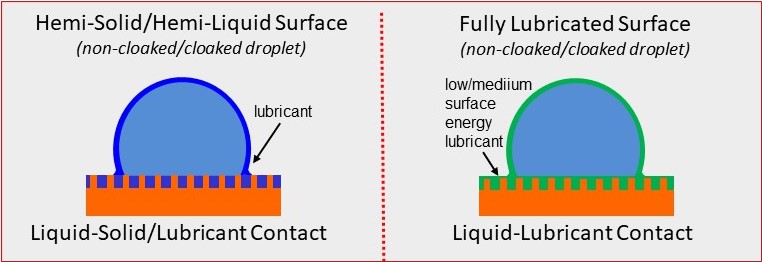
However, whereas the pitcher plants have surfaces that are lubricated by water and repel oil, the artificial surfaces can be lubricated by oil and repel water. By using perflorinated fluids as the wetting lubricant, it is possible to create omniphobic surfaces that repel most things. Water droplets do not “ball up” and roll off these super-slippery surfaces. Nevertheless, water is readily shed when the surface is tilted by even a small amount.
SLIP surfaces are inherently self-healing, and uses include anti-graffiti spray paint and slippery ice trays. Useful information on SLIPS from the laboratory of Professor Aizenberg at Harvard, including a set of videos showing “SLIPS in action”, can be found here.
